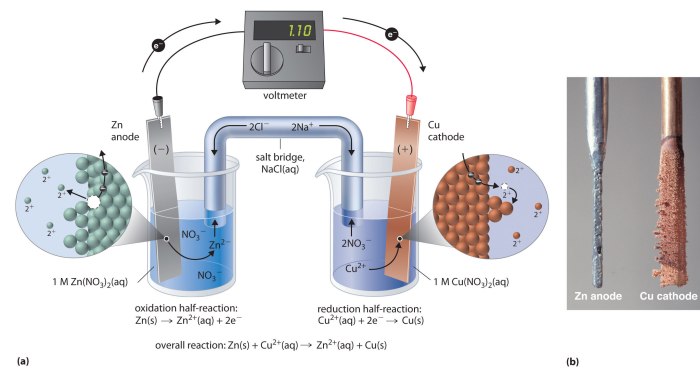
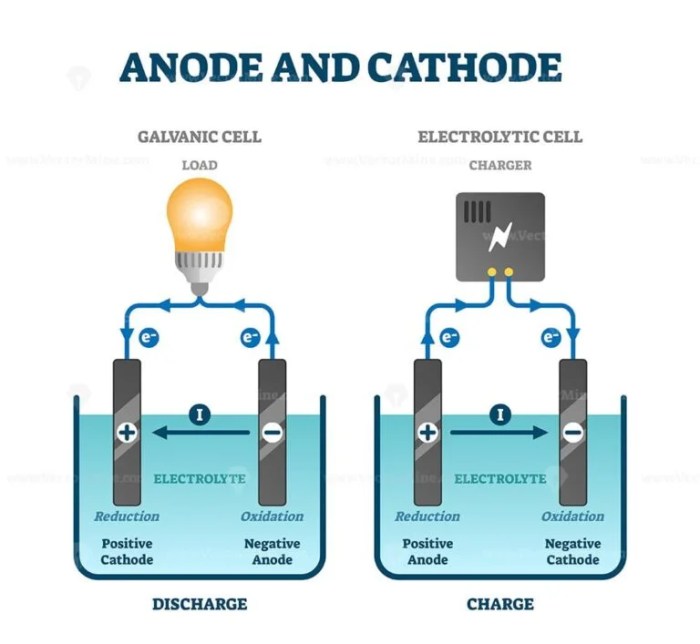
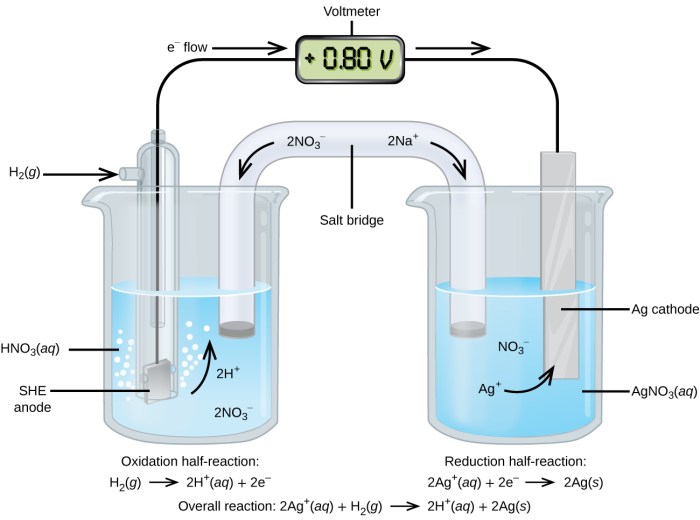
Describe the electrodes in this tin copper galvanic cell – In a tin-copper galvanic cell, the electrodes play a crucial role in the electrochemical process. This article explores the materials, design, placement, surface modifications, and connections of the tin and copper electrodes, providing a comprehensive understanding of their significance in the cell’s operation.
The tin and copper electrodes are essential components of the galvanic cell, enabling the conversion of chemical energy into electrical energy. Their unique properties and configurations contribute to the cell’s efficiency and performance.
Electrodes in a Tin-Copper Galvanic Cell
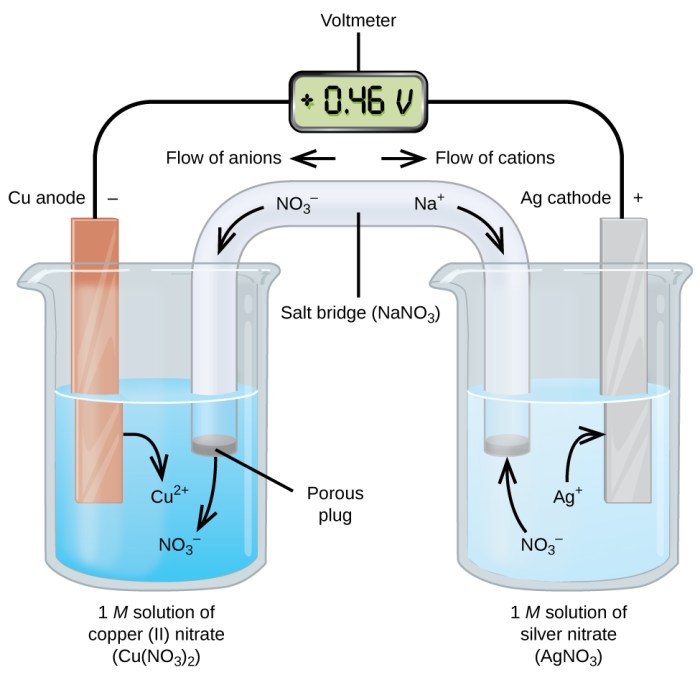
Electrodes play a crucial role in the electrochemical process of a tin-copper galvanic cell. Tin and copper are selected for their specific properties that contribute to the efficient generation of electricity.
Electrode Materials
- Tin Electrode:The tin electrode acts as the anode, where oxidation occurs. Tin is chosen for its low oxidation potential, making it prone to losing electrons and forming tin ions (Sn 2+).
- Copper Electrode:The copper electrode serves as the cathode, where reduction takes place. Copper has a high reduction potential, readily accepting electrons and reducing copper ions (Cu 2+) to form metallic copper.
Electrode Design, Describe the electrodes in this tin copper galvanic cell
The physical characteristics of the electrodes influence the cell’s performance:
- Size and Shape:Larger electrodes provide a greater surface area for electrochemical reactions, increasing current flow.
- Surface Area:The surface area of the electrodes affects the number of active sites available for electron transfer, impacting the cell’s efficiency.
Electrode Placement
Optimal electrode placement is crucial for efficient cell operation:
- Distance:The distance between the electrodes affects the resistance of the cell. Closer electrodes reduce resistance and facilitate current flow.
- Orientation:The orientation of the electrodes can influence the direction of current flow and the efficiency of the cell.
Electrode Surface Modifications
Surface modifications can enhance electrode performance:
- Increasing Surface Area:Modifications like roughening or coating the electrodes can increase their surface area, providing more sites for electrochemical reactions.
- Enhancing Catalytic Activity:Applying catalysts to the electrode surfaces can accelerate the electrochemical reactions, improving the cell’s efficiency.
Electrode Connections
Proper electrode connections are essential for efficient current flow:
- Electrical Contacts:The electrodes are connected to the external circuit using conductive materials to ensure proper electrical contact.
- Minimizing Resistance:Connections should be designed to minimize resistance, allowing for efficient transfer of electrons.
Questions and Answers: Describe The Electrodes In This Tin Copper Galvanic Cell
What is the significance of the tin and copper electrodes in a galvanic cell?
The tin and copper electrodes serve as the anode and cathode, respectively, in a galvanic cell. The tin electrode undergoes oxidation, releasing electrons that flow through the external circuit, while the copper electrode undergoes reduction, accepting electrons from the circuit.
How does the design of the electrodes affect the cell’s performance?
The size, shape, and surface area of the electrodes influence the rate of electrochemical reactions. Larger surface areas provide more active sites for reactions, while specific shapes can optimize the flow of ions and electrons.
Why is the placement of the electrodes important?
The distance and orientation of the electrodes affect the resistance of the cell and the efficiency of the electrochemical process. Optimal placement minimizes resistance and maximizes the interaction between the electrodes.
What are the benefits of surface modifications to the electrodes?
Surface modifications, such as increasing surface area or enhancing catalytic activity, can improve the performance of the electrodes by promoting faster reaction rates and reducing overpotential.
How are the electrodes connected to the external circuit?
The electrodes are typically connected to the external circuit using conductive wires or plates. Proper connections ensure efficient current flow and minimize energy losses.